Jim C Bradley
Member
- Messages
- 4,945
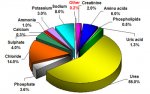
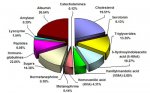
" (indeed this is one step in the "rust bluing" process that was historically used to put a protective coat on firearms - they also traditionally used urine to create the flash rust on the polished metal.. amazing what urine was used for back then.. steel bluing, dying - traditional indego was done in a urine base, hide tanning, gunpowder, and even tooth whitening!  )"
)"
Did you know that urine was the first organic compound artificially produced? Ah, the romance of history.
Enjoy,
JimB
Did you know that urine was the first organic compound artificially produced? Ah, the romance of history.
Enjoy,
JimB